AIP Patients Show Enlarged Brain Cavities, Reduced Brain Blood Flow, Study Suggests
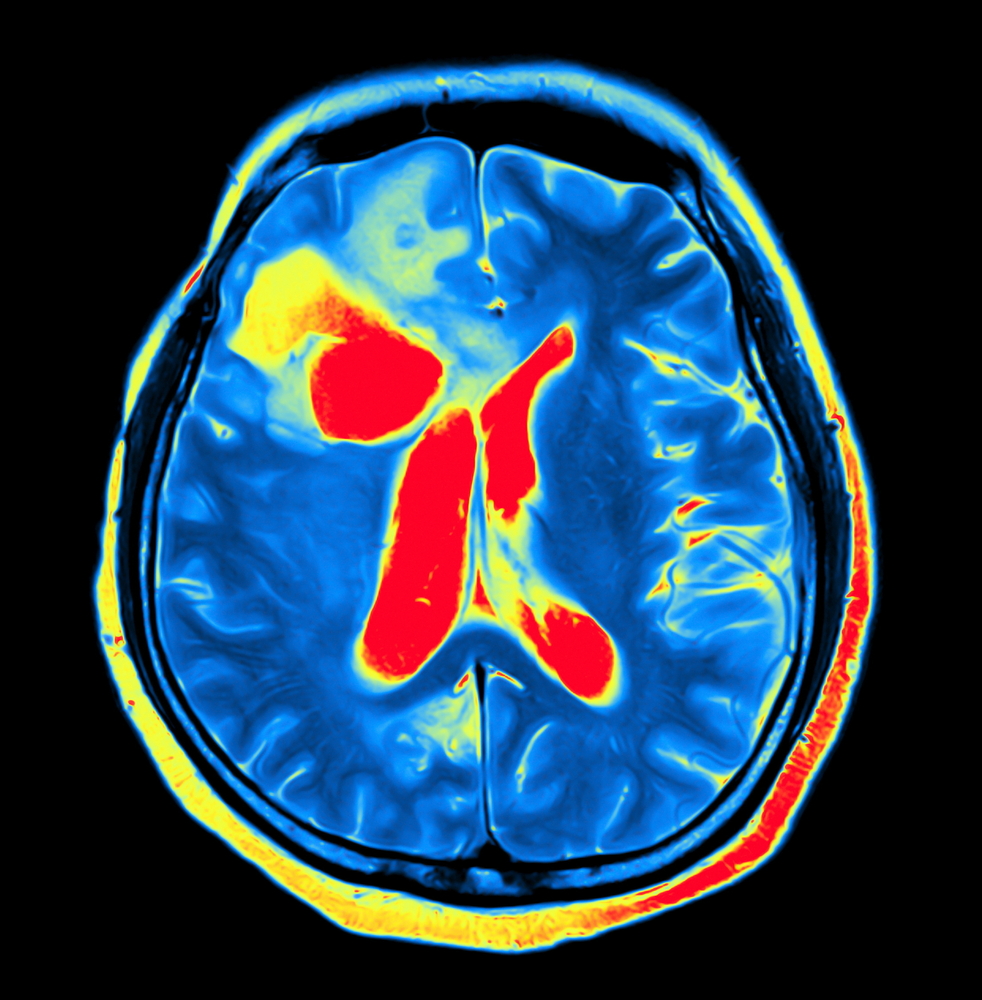
People with acute intermittent porphyria (AIP) have structural changes in the brain, namely an enlargement of the brain’s ventricles — a set of four communicating cavities — associated with a reduction in the brain’s blood flow, a study suggests.
A liver-targeted gene therapy designed to restore the levels of porphobilinogen deaminase (PBGD), the defective enzyme in people with the rare metabolic disorder, protected against these brain changes in a mouse model of AIP.
The study, “Brain ventricular enlargement in human and murine acute intermittent porphyria,” was published in the journal Human Molecular Genetics.
Deficient activity of PBGD, also known as hydroxymethylbilane synthase, causes the accumulation of porphyrins, which are required for the production of heme — a molecule essential for the transport of oxygen in red blood cells and the breakdown of certain compounds in the liver.
AIP causes severe abdominal pain that is often accompanied by gastrointestinal complications such as nausea, vomiting, and constipation. Certain patients also develop neurological symptoms, including seizures, confusion, insomnia, anxiety, and depression.
The condition belongs to a group of disorders known as the porphyrias, all due to the deficiency of enzymes essential to heme’s creation.
Based on previous studies suggesting the narrowing of blood levels, called vasoconstriction, in AIP patients, a group of Spanish researchers asked whether the disorder causes changes in the brain’s structure.
To learn more, the team used MRI scans to analyze the brain structures of eight patients with severe AIP who were experiencing recurrent acute attacks, defined as those requiring two or more hospitalizations in the previous year.
The participants were comprised of two men and six women, with a mean age of 46.6 years, enrolled from a prior clinical trial (NCT02076763). They had no epileptic seizures and did not have posterior reversible encephalopathy syndrome (PRES), a condition that can co-occur with AIP. PRES is linked with high blood pressure, and symptoms include headache, seizures, and altered mental status.
Results showed that the total brain volume of the AIP patients was similar to that of 16 age- and sex-matched healthy people (controls).
However, ventricular brain volume (VBV) was significantly larger in the AIP patients than in the controls, as was the percentage of the ventricular by total brain volume. A higher percentage was associated with a lower number of heme injections. Notably, the ventricular system in the brain is a set of communicating cavities, or ventricles, that contain cerebrospinal fluid, which also surrounds the brain and spinal cord.
All of the AIP patients showed increased blood levels of endothelin-1 (EDN1) compared with both the controls and with eight asymptomatic AIP carriers, with normal levels of porphyrin precursors in the urine. END1 plays an important role in vasoconstriction and has been associated with liver disease.
Next, the scientists measured cerebral blood flow (CBF) — the blood supply to the brain in a given period of time — in two patients.
In a 47-year-old woman, CBF was reduced by 6.2% within seven months of a first measurement of the blood flow. At the second measure, the woman was recovering from an acute attack, as shown by high levels of porphyrin precursors in the urine.
The second patient, a 63-year-old woman, had been hospitalized due to an acute attack immediately before the first CBF measurement. Eight years later, after experiencing clinical improvements, the woman’s CBF measure had increased by 22.1%.
“These results suggest that CBF decreases during acute attacks of porphyria perhaps due to increased levels of porphyrin precursors,” the researchers wrote.
The team then investigated whether the changes in brain structure and circulation also were maintained in a mouse model of AIP.
As in the human patients, these mice showed brain ventricular enlargement, although the increases in porphyrin precursors were not as severe as those seen in the people with AIP.
The mice then were given phenobarbital, which mimics porphyria attacks, over a course of eight months. The barbiturate exacerbated the enlargement of the brain’s ventricles in these mice.
Next, the investigators used a gene therapy, known as rAAV-PBGD, to restore the production of PBGD in the liver. This treatment prevented the enlargement of the brain ventricles and the accumulation of heme precursors in the AIP mouse model.
Brain tissue analyses showed no signs of lack of blood supply or death of nerve cells after three phenobarbital administrations, suggesting that the “enlarged ventricular size found in AIP mice cannot be related to brain atrophy [shrinkage] or neuronal cell loss,” the team wrote.
These mice also showed a 39% reduction in CBF compared with healthy mice, which was rescued with the gene therapy.
Overall, “in the brain of patients with severe AIP as well as in AIP mice, we found cerebral ventricle enlargement seemingly as a result of vascular dysfunction,” the researchers wrote. “Liver-directed gene therapy protected against the morphological consequences of the disease seen in the brain of AIP mice.”






